- You are here:
- Home »
- Disorder »
- Boltzmann Entropy
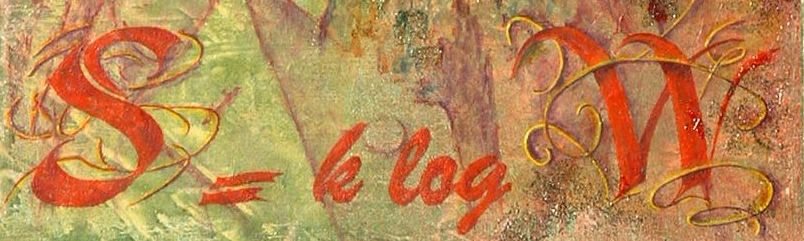
Boltzmann Entropy

Ludwig Boltzmann was able to demonstrate the exceptional relationship: S=k ln W
It means that entropy S is bound to the disorder which reigns in the system of molecules.
This relationship is remarkable in more than one way. It builds a bridge between our macroscopic world and microscopic world, in which molecules are agitated continuously.
How to explain the emergence of irreversibilities (disorder, entropy) observed in our macroscopic world from laws which ignore them at the microscopic level?
It is the basis of H theorem of Boltzmann, yet fiercely discussed nowadays.
We give below an experimental demonstration of the Boltzmann equation.
About the Author Michel Pluviose
Michel Pluviose is Honorary Professor of Le Conservatoire National des Arts et Métiers (CNAM), and formerly Chair of Turbomachines who received his Doctor of Sciences from Université Pierre et Marie Curie - Paris VI. A hands-on engineer, Michel has worked with leading institutions, including as an engineer at Hispano-Suiza, SNECMA, Head of the laboratory at ATTAG (Association technique pour les turbomachines et turbines à gaz), Manager for compressible fluid activities at CETIM (Centre technique des industries mécaniques), and manager of the treaty « Machines hydrauliques et thermiques » at the « Techniques de l’Ingénieur » publications.
Related Posts
Aircraft carrier Charles-de-Gaulle: Safety Valve Incident (October 15th, 2010)
Fessenheim Power Plant (april 2014)
Experimental demonstration of Boltzmann equation.
The principle of worst action allows the escape chaos
The principle of worst action applied to valves
The principle of worst action applied to a perforated plate.
The principle of worst action

